Best Lewis Structure for Clo3
2 Identify the number of electrons that. 1 First count the number of valence electrons present in the outermost shell of each atom present in the molecule.
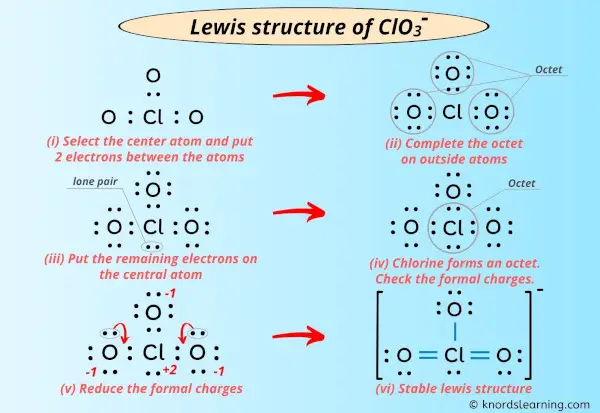
Lewis Structure Of Clo3 With 6 Simple Steps To Draw
The best Lewis structure for chlorate ClO3 has an expanded octet as shown below.

. Assign formal charges to the elements in each of the structures below. ClO3- Lewis Structure Molecular Geometry Hybridization S. We can draw three inequivalent Lewis structures for the chlorate ion CIO3.
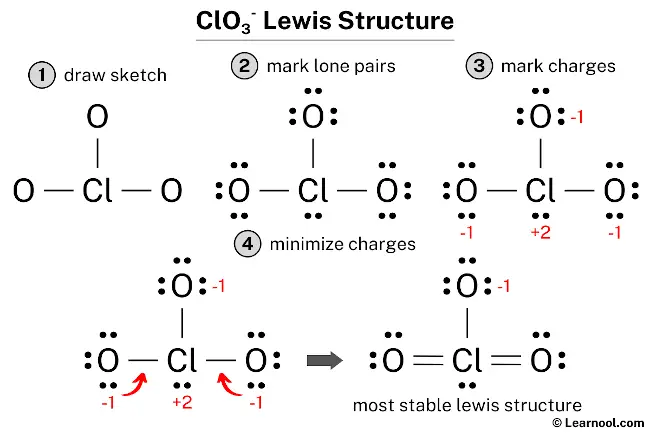
And the single bonded oxygen atom has -1 formal charge. Thus the total charge of the molecule -1 as before but now only one atom is not neutral which is more favorable than the structure originally proposed. Of electrons No.
Formal charge valence electrons No. In ClO3 molecule central chlorine atom has one lone pair and molecular geometry is trigonal bipyramidal. A step-by-step explanation of how to draw the ClO3- Lewis Structure Chlorate Ion.
Total valence electrons of oxygen and chlorine atoms and negative charge are considered to draw the ClO 3- lewis structure. Also the above structure is more stable than the previous structures. Clo3 Lewis Structure Chlorate Ion Molecules Lewis Electrons.
Therefore this structure is the most stable lewis structure of ClO 3. The Lewis structure for ClO 3- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. Brady The Molecular Nature of Matter 6th Edition Copia.
Formal charge in oxygen 6-4-2 0. This tutorial will help you deal with the lewis structure and molecular geometry for chlorate ClO3-. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation.
The Chlorine atom Cl is at the center and it is surrounded by 3 Oxygen atoms O. Lewis Structure of nitrite ion. We start with a valid Lewis structure and then follow these general rules.
And in this case the most electronegative element is oxygen. 08649 06011 03109 06163 12325 06426 03280 04978. The Chlorine atom has 1 lone pair.
Molecular mass of ClO3 is 8345gmol. The total valence electron is 26 for drawing ClO3 Lewis structure. For the ClO3 - resonance structure there are two equivalent.
This chemistry video tutorial explains how to draw the lewis structure of the Chlorate ion ClO3-My Website. Draw all relevant resonance structures for chlorate using the correct notation AND write 1-2 sentences to describe the meaning of these resonance structures in terms of bond length. Lewis Structure For Clo3 - 13 images - ch3o lewis structure how to draw the lewis structure for clo4 lewis structure how to draw the lewis structure asf6 lewis structure how to draw the lewis structure for lewis dot structure of clo2 chlorite ion youtube.
Lewis Structure of nitrite ion. Respond to Questions on Lewis structure and molecular geometry using VSEPR model. Lewis Structure of ClO 3- Chlorate ion Lewis structure of ClO 3- ion is drawn step by step in this tutorial.
Lewis structure for clo3- - The concept used to solve this problem is based upon the Lewis structures. This is okay because the structure with a negative charge on the most electronegative atom is the best lewis structure. Remember Chlorine is in Period 3 and can hold more than 8 valence electrons.
Valence electrons in ClO3. The Best 13 Clo2 Lewis Structure - layengijun. When we drew the lewis structure overall charge of the ion should be -1.
Chemistry learning made easy. The lewis structure of ClO 3 contains two double bonds and one single bond with chlorine in the center and three oxygens on either side. Count oxygen atoms starting from the left for each structure.
The formal charge of chlorate anion is calculated by considering its Lewis structure. Lewis Structure For Clo3 Sources. Lewis Structure of ClO 3- Chlorate ion Lewis structure of ClO 3- ion is drawn step by step in this tutorial.
The ClO3- Lewis structure is a good structure to help you understand w. When we drew the lewis structure overall charge of the ion should be -1. Lewis structure of ClO3- or Chlorate ion contains two double bonds and one single bond between the Chlorine Cl atom and Oxygen O atom.
You might think youve got the correct Lewis structure for ClO 3- at first. These structures can NOT conform to any molecular geometry too much strain. Formal charge in one oxygen with single bond 6-6-1 -1.
There are two resonance structures ClO3 - Chlorate ion. It is commonly known as chlorate having chemical formula ClO3. Formal charge of chlorine 7-2-5 0.
These are the following rules for drawing the Lewis structure of a compound. The left oxygen atom and right oxygen atom have two lone pairs the top oxygen atom has three lone pairs and the chlorine atom has one lone pair. Total valence electrons of oxygen and chlorine atoms and negative charge are considered to draw the ClO 3- lewis structure.

Lewis Structure Of Clo3 Chlorate Ion
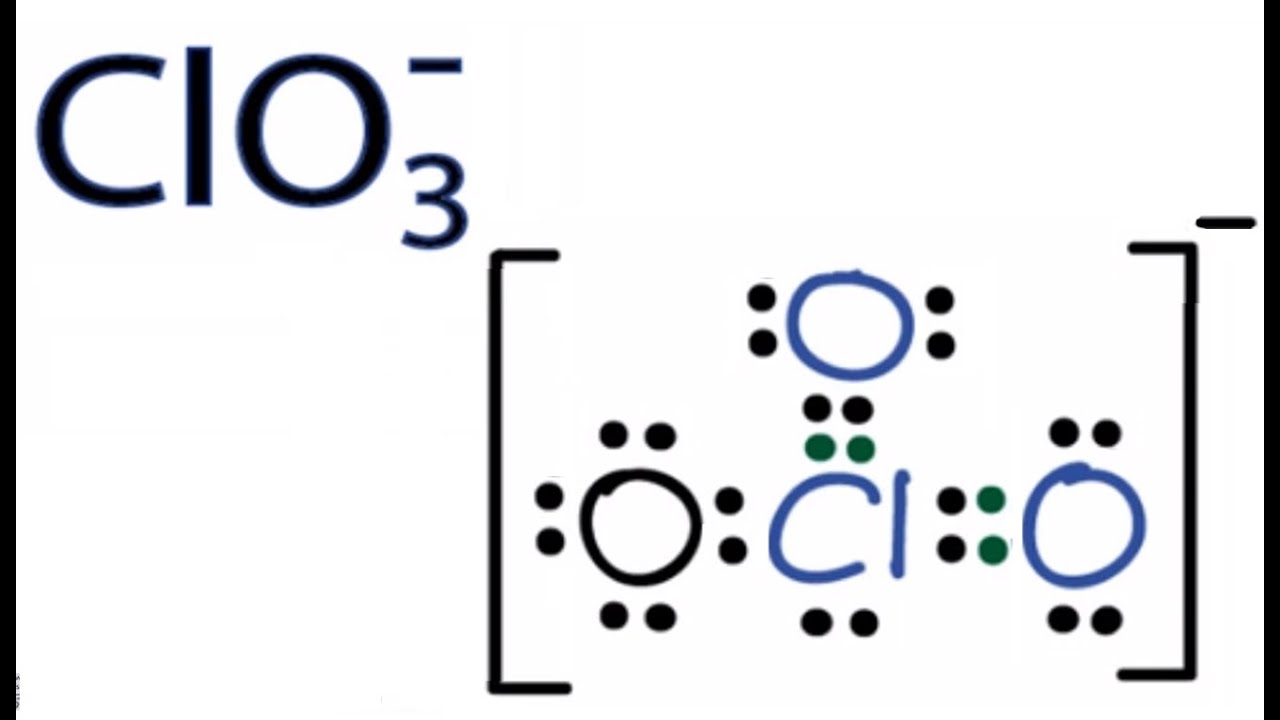
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube
No comments for "Best Lewis Structure for Clo3"
Post a Comment